Alzheimer’s research is at the forefront of the battle against one of the most challenging neurodegenerative diseases affecting millions. Under the guidance of pioneering neuroscientist Beth Stevens, the investigation into microglial cells, which act as the brain’s immune system, has unveiled crucial insights into how these cells can both protect and harm neural circuits. By examining the role of microglia in processes like synaptic pruning, Stevens’ work has opened up new avenues for potential Alzheimer’s treatment, aiming to prevent the detrimental effects of aberrant pruning on cognitive health. As the population ages, ongoing studies are increasingly vital; the Alzheimer’s Association estimates the number of cases may double by 2050, highlighting the urgent need for effective interventions. With a focus on foundational science, Stevens’ research not only enhances our understanding of these disorders but also lays the groundwork for future therapeutic strategies.
Investigating Alzheimer’s disease encompasses a variety of interconnected terms and concepts, including cognitive decline and memory loss associated with aging. This field of study highlights the critical role of the brain’s immune defense mechanisms, particularly those performed by microglial cells, which are essential in maintaining neural health and stability. The work of researchers like Beth Stevens emphasizes the significance of understanding how these immune cells can influence the progression of diseases affecting the nervous system. This research is not only fundamental for developing new diagnostic tools but also for creating innovative treatments to slow or even reverse the effects of neurodegenerative conditions. By delving into the complexities of brain function and immune interactions, researchers are paving the way for breakthroughs in the management of Alzheimer’s and related diseases.
Understanding Microglial Cells in Alzheimer’s Disease
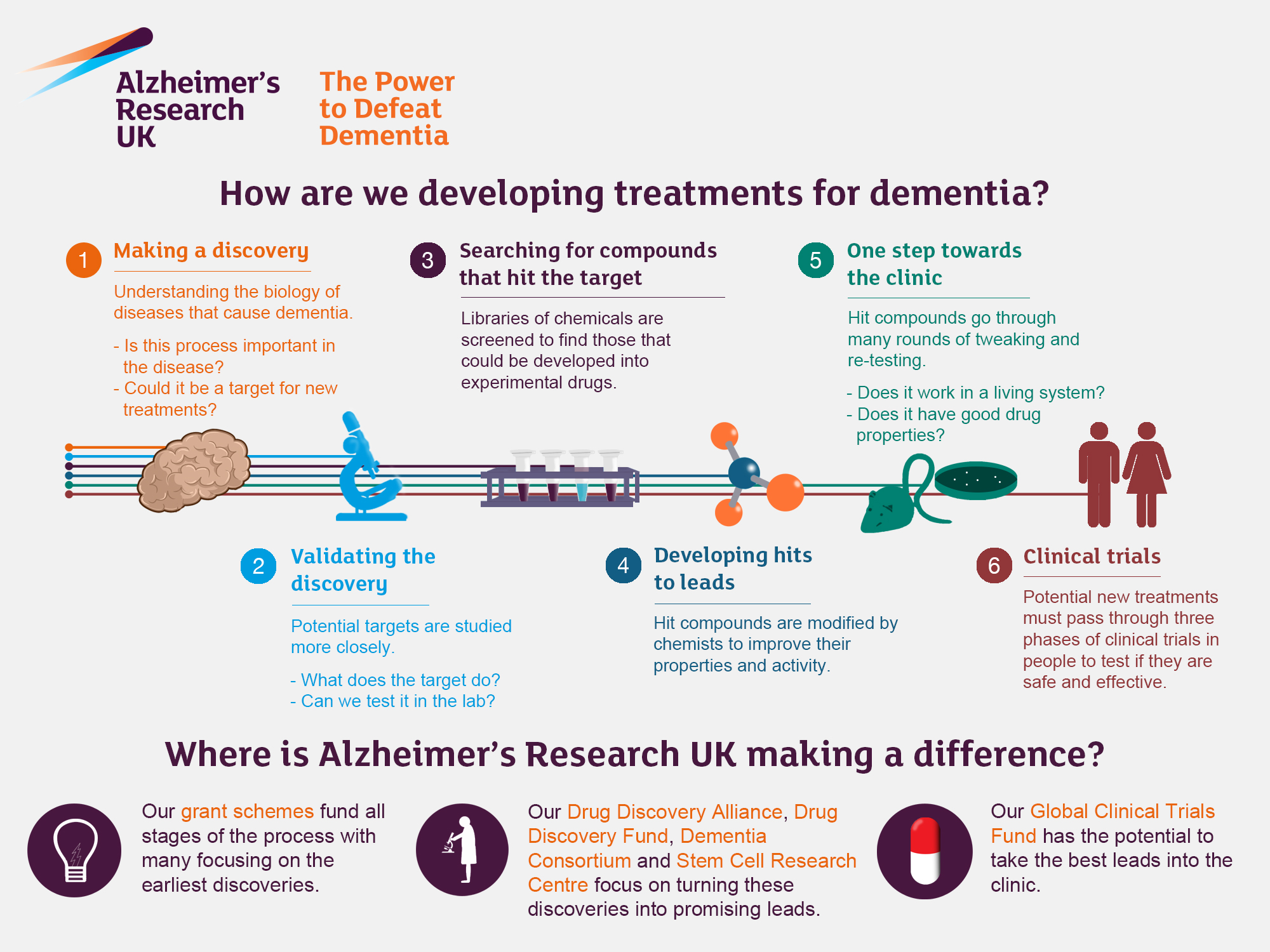
Microglial cells play a crucial role in the central nervous system, functioning as the brain’s immune system. They are responsible for monitoring the health of neurons and maintaining homeostasis within the brain. In conditions like Alzheimer’s disease, microglia can become overactive or dysregulated, leading to incorrect synaptic pruning and the exacerbation of neurodegeneration. This understanding highlights the importance of targeting microglial activity in developing effective Alzheimer’s treatments.
Research by Beth Stevens emphasizes the dual role of microglial cells in both protecting and damaging the brain’s neural architecture. Her studies at Boston Children’s Hospital reveal that while these cells help eliminate dead neurons and damaged synapses, aberrant pruning can contribute to the onset of Alzheimer’s and other neurodegenerative diseases. Such findings underline the potential for therapies that can modulate microglial behavior to protect neural circuits and alleviate symptoms of Alzheimer’s.
The Impact of Alzheimer’s Research on Treatment Strategies
Alzheimer’s research is crucial as it shapes treatment strategies for millions affected by this disease. Innovations in understanding the cellular mechanisms behind Alzheimer’s, particularly regarding microglial cells, can lead to targeted therapies aimed at interrupting the neurodegenerative process. For instance, new biomarker discovery based on microglial activity could facilitate earlier diagnosis and treatment interventions, greatly enhancing the quality of life for patients.
With an aging population, the need for effective Alzheimer’s treatments is more pressing than ever. The projections by the Alzheimer’s Association underscore the urgent need for advancements in research to reduce the incidence of this debilitating condition. By tackling the pathophysiology of Alzheimer’s through thorough research and innovative approaches, scientists like Beth Stevens are paving the way for future therapies that could redefine care and treatment in the years to come.
Greener research funding landscapes play a significant role in advancing Alzheimer’s treatment research. The ongoing support from organizations like the National Institutes of Health (NIH) empowers researchers like Stevens to investigate complex brain mechanisms. Their findings are not just academic; they represent hope for breakthrough treatments that could impact millions suffering from Alzheimer’s disease as they age.
Frequently Asked Questions
What is the role of microglial cells in Alzheimer’s research?
Microglial cells act as the brain’s immune system, playing a crucial role in Alzheimer’s research by detecting and responding to signs of illness or injury, such as clearing damaged cells and pruning synapses. Abnormal microglial activity has been linked to neurodegenerative diseases like Alzheimer’s, making them a focus of current studies aimed at understanding and potentially mitigating these conditions.
How does Beth Stevens contribute to Alzheimer’s treatment through her research?
Beth Stevens contributes to Alzheimer’s treatment by studying microglial cells and their role in synaptic pruning. Her research has revealed how improper pruning can lead to neurodegenerative diseases. By identifying these processes, her work paves the way for developing new Alzheimer’s treatments and early detection biomarkers, fundamentally enhancing our understanding of brain health.
Why is understanding neurodegenerative diseases important in Alzheimer’s research?
Understanding neurodegenerative diseases is vital in Alzheimer’s research because it helps uncover the mechanisms behind brain degeneration, such as those involving microglial cells. This knowledge is essential for developing effective therapies and interventions aimed at slowing down or preventing Alzheimer’s disease, thus improving the quality of life for millions.
What potential impact does microglial research have on Alzheimer’s disease prevention?
Microglial research has significant potential impacts on Alzheimer’s disease prevention by revealing how these immune cells interact with neurons. Understanding the mechanisms of microglia in synaptic pruning may lead to identifying risk factors or early biomarkers for Alzheimer’s, enabling preventative strategies and treatments that could substantially reduce the disease’s burden.
How does the aging population affect Alzheimer’s research and treatment?
The aging population increases the urgency of Alzheimer’s research and treatment, as the number of cases is projected to double by 2050. This demographic shift necessitates advancements in understanding the role of microglial cells and neurodegenerative mechanisms, ensuring that effective treatments and care strategies keep pace with the growing population of affected individuals.
| Key Points | Description |
|---|---|
| Research Focus | Beth Stevens studies microglial cells in the brain’s immune system. |
| Role of Microglia | Microglia clean up dead cells and prune synapses, but improper pruning can lead to diseases like Alzheimer’s and Huntington’s. |
| Impact on Alzheimer’s | Research could lead to new medicines and biomarkers for earlier detection, vital for the 7 million Americans with Alzheimer’s. |
| Funding Importance | Stevens’ research greatly benefited from federal funding, especially from the National Institutes of Health (NIH). |
| Long-Term Vision | Basic science allows for new discoveries that can ultimately improve treatment and understanding of human diseases. |
Summary
Alzheimer’s research remains a critical field focusing on innovative discoveries that could change lives. The groundbreaking work by Beth Stevens on microglial cells highlights how the brain’s immune response can influence neurodegenerative diseases. By understanding and manipulating these immune cells, researchers aim to develop new treatments and early detection methods for the millions affected by Alzheimer’s. With the aging population and increasing prevalence of this disease, fostering such research is essential for future healthcare improvements.