Medical research oversight plays a critical role in ensuring that studies involving human participants are conducted ethically and safely. As funding cuts threaten the financial backbone of research programs, the impact on patient safety in studies becomes increasingly concerning. The decline in federal research grants, particularly from the NIH, directly affects the capacity of Institutional Review Boards (IRBs) to uphold research ethics and protect the rights of those involved. Harvard Medical Research has historically led initiatives to promote rigorous oversight through systems like SMART IRB, which streamlines review processes across multiple sites. However, with diminishing resources, the essential functions of IRBs are jeopardized, potentially leaving patients vulnerable during critical research phases.
The governance of medical investigations has never been more crucial, as oversight mechanisms safeguard the integrity of clinical trials and human well-being. Within the framework of this oversight, the function of IRBs becomes paramount, as they navigate regulatory landscapes to protect research participants. The ramifications of decreased financial support not only jeopardize research ethics but also increase the risk of adverse outcomes during studies. The dilapidation of funding streams, like those faced by Harvard Medical Research, amplifies concerns about comprehensive oversight necessary for patient safety. As society continues to wrestle with the ethics of our medical advancements, it becomes imperative to address how we can sustain these vital oversight systems.
The Critical Role of IRBs in Safeguarding Research Participants
Institutional Review Boards (IRBs) play a pivotal role in ensuring the ethical conduct of medical research. By meticulously reviewing research proposals, they help to protect participants’ rights and welfare. This oversight is crucial for the compliance of studies with various laws and regulations, ensuring a balance between scientific advancement and ethical responsibility. The IRB’s functions include evaluating study designs, recruitment strategies, and informed consent processes, addressing risks, and implementing data monitoring protocols. Without IRBs, the integrity of clinical research would be jeopardized, potentially exposing participants to undue harm.
IRBs serve not just as regulators but as advocates for participant safety in studies. They ensure that potential risks are clearly communicated and that participants have the necessary information to make informed decisions. Moreover, their involvement extends beyond initial approval; they monitor ongoing research to ensure compliance with ethical standards throughout the study’s duration. This vigilant oversight fosters trust in the research process and reassures the public about the commitment to patient safety in medical studies.
Impact of Research Funding Cuts on Patient Safety
Recent funding cuts, such as the freeze on $2 billion in federal research grants to institutions like Harvard, can have dire consequences for patient safety in medical research. These financial constraints limit the ability of IRBs to conduct thorough reviews and oversight of studies, thereby increasing the risk of ethical lapses. Research processes may be rushed or inadequately monitored, leading to potential safety risks for participants. The halt of funding not only stifles innovative research but also undermines the ethical frameworks that protect participants, as diminished resources can lead to lapses in necessary monitoring and support.
Furthermore, the cancellation of grants can disrupt ongoing studies, preventing new clinical sites from joining essential research projects. Such interruptions can have cascading effects, jeopardizing participant safety and eroding public trust in the research system. As studies are delayed or abandoned, the voices of participants, particularly those with critical health conditions, are sidelined, and opportunities for scientific progress are lost. The healthcare community, including researchers and IRBs, must advocate for sustained funding to ensure robust oversight and, ultimately, the safety of individuals participating in these vital studies.
The Ethical Imperative of Research Oversight
The ethical framework surrounding medical research is rooted in historical lessons that highlight the consequences of neglecting participant rights. Events like the Tuskegee Study and the Willowbrook hepatitis research illustrate the need for rigorous oversight and accountability in research practices. Institutional Review Boards were established in response to these historical injustices, emphasizing the necessity of protecting human subjects in research. Today, this ethical imperative drives IRBs to ensure that research is conducted with the utmost respect for participants’ autonomy and safety.
Maintaining ethical standards in medical research not only safeguards participants but also fosters public trust, which is essential for the continued advancement of science. As stakeholders in the research process, IRBs are charged with the responsibility of instilling confidence in the system. Their work includes evaluating the implications of funding decisions on research ethics, ensuring that financial pressures do not compromise the quality of oversight required to protect human subjects. A resurgence in addressing these ethical concerns must occur, especially in light of increasing budget cuts that threaten the integrity of the research landscape.
Funding as a Catalyst for Ethical Oversight in Medical Research
Adequate funding is crucial for maintaining the ethical oversight of medical research studies. When financial resources are plentiful, IRBs can perform their functions effectively, reviewing proposals comprehensively and providing the necessary support to researchers. Funding enables institutions to invest in training and resources that enhance the capabilities of IRBs, equipping them to address ethical challenges proactively. Conversely, cuts to research funding impede this progress, leading to a diminished capacity for oversight and increased vulnerability for research participants.
The intersection of funding and research ethics underscores the need for sustained investment in the research ecosystem. Healthy funding streams empower institutions to adopt best practices in the ethical conduct of research. Additionally, they enable collaboration among multiple sites through systems like the SMART IRB, which streamline the approval process and enhance participant protections across various studies. Recognizing the relationship between financial backing and ethical oversight is imperative; as research institutions face budget cuts, it becomes increasingly essential to advocate for funding that prioritizes participant safety and ethical integrity.
Advancing Patient Safety Through Collaborative Research
Collaboration among research institutions is essential to advancing patient safety in medical studies. Initiatives like the SMART IRB facilitate the sharing of oversight responsibilities across multiple sites, allowing for streamlined processes that uphold ethical standards while promoting innovation. The collaborative model not only enhances the efficiency of studies but also strengthens the protective measures in place for participants. By reducing redundancies in IRB reviews, research teams can focus on ensuring that participant welfare remains a paramount concern.
Moreover, collaborative research efforts foster a culture of shared accountability, where institutions work together to develop and refine ethical standards. This framework encourages ongoing dialogue about challenges and best practices in participant protection, ultimately benefiting the research community as a whole. When institutions partner effectively, they enhance their collective ability to advocate for participant rights, ensuring that patient safety is prioritized, even amidst funding challenges. Collaboration becomes a powerful tool in driving ethical research and reinforcing public trust.
Lessons from the Past: The Importance of Ethical Compliance
Historical events remind us of the importance of ethical compliance in medical research. The dark chapters of human experimentation underscore the necessity of having rigorous safeguards in place to protect participants. Advances in ethical guidelines have been informed by the lessons learned from past atrocities, leading to the establishment of IRBs as crucial components of the research process. These boards ensure that the rights and welfare of participants are prioritized in today’s research landscape, reflecting a commitment to prevent history from repeating itself.
As we navigate the complexities of modern medical research, it is crucial to remember the foundations laid by these historical lessons. Compliance with ethical standards is not merely a bureaucratic obligation; it is a moral imperative that underscores the sanctity of human life and dignity. Research institutions must remain vigilant against the risks posed by funding cuts, as a failure to prioritize ethical compliance can erode the hard-won trust of the public and participants alike. Continuous ethics training and public engagement are essential to uphold the integrity of research and ensure that participant safety remains at the forefront.
The Relationship Between Medical Funding and Patient Trust
The relationship between funding levels and public trust in medical research cannot be overstated. When funding is stable, research institutions can demonstrate their commitment to conducting studies ethically and with the utmost diligence towards participant safety. However, funding cuts create an environment of uncertainty, where institutions may struggle to maintain the rigorous oversight required by IRBs. The perception that financial pressures compromise research integrity can lead to public skepticism and diminish the willingness of individuals to participate in clinical trials.
Building and maintaining trust in the research enterprise is essential for its success, particularly as society faces pressing health challenges. A transparent approach to funding and accountability is necessary to reassure participants that their safety is the priority. Institutions must communicate openly about how funding impacts the oversight of research, as well as the measures taken to protect participants despite financial constraints. Engaging with the community and fostering dialogue about the importance of ethical research can help bridge gaps and rebuild trust.
The Role of Legislative Support in Research Oversight
Legislative support plays a vital role in reinforcing the oversight of medical research. Bills and policies that prioritize funding for research institutions and safeguard participant rights are essential components of a robust research framework. Lawmakers must recognize the implications of budget cuts on both patient safety and the ethical conduct of research. By advocating for policies that secure ongoing funding and support for IRBs, legislators can help ensure that research institutions have the resources necessary to uphold the highest ethical standards.
Moreover, legislative initiatives can establish guidelines for ethical oversight that all institutions must adhere to. This consistent regulatory framework fosters a culture of compliance and accountability across the research landscape. It is critical for policymakers to engage in dialogue with research institutions, practitioners, and the public to understand the ethical challenges faced in the field. Through collaborative policymaking, we can create an environment where ethical research is prioritized and funded adequately, ultimately benefiting patient safety and the integrity of scientific inquiry.
Strategies for Strengthening Research Ethics Amidst Funding Cuts
Strengthening research ethics amidst funding cuts is a multifaceted challenge that requires proactive strategies. First and foremost, institutions must prioritize efficient allocation of existing resources, ensuring that IRB functions are not undermined by financial constraints. Training programs for IRB members and researchers should be established to enhance their understanding of ethical oversight in the face of budget limitations. Such initiatives can empower staff to maintain high ethical standards while navigating the complications posed by diminished funding.
Engaging with stakeholders, including community representatives and advocacy groups, can also enhance the ethical landscape of research. By incorporating diverse perspectives into the research design and oversight processes, institutions can ensure that participant safety and rights are adequately addressed. Cultivating a robust dialogue with the communities being studied can build trust and promote transparency, reassuring participants that their welfare is a primary concern. In this way, collaborative efforts can fortify the ethical foundations of research even amidst challenging financial climates.
Frequently Asked Questions
What is the role of IRBs in medical research oversight?
Institutional Review Boards (IRBs) play a pivotal role in medical research oversight by ensuring that research involving human participants is ethically conducted. They review research proposals to assess potential risks, enforce informed consent processes, and safeguard the rights and welfare of participants, thus maintaining the integrity of research and patient safety.
How do funding cuts impact patient safety in medical research?
Funding cuts disrupt the essential oversight functions of research bodies like IRBs, potentially compromising patient safety. A lack of resources can lead to reduced monitoring of studies, delays in ethical reviews, and inadequate training for researchers, all of which may increase risks for participants in clinical research.
What are the implications of IRB oversight for research ethics?
IRB oversight is crucial for upholding research ethics, as it enforces compliance with ethical standards and regulations. By reviewing study protocols, IRBs ensure that participant welfare is prioritized, informed consent is obtained, and that studies do not exploit vulnerable populations, thus fostering trust in the research process.
How does SMART IRB facilitate multi-site research?
SMART IRB streamlines the process of medical research oversight across multiple sites by allowing a single IRB to coordinate reviews for collaborative studies. This reduces administrative burdens, speeds up the research process, and ensures consistent ethical oversight, crucial for patient safety and effective research outcomes.
What historical events led to the development of IRBs and medical research oversight?
IRBs were established in response to historical abuses in medical research, such as the Tuskegee Syphilis Study and unethical experiments during World War II. These events highlighted the need for rigorous ethical oversight to protect participants, leading to the formation of IRBs and regulations ensuring informed consent and patient welfare.
What are the consequences of halted research due to funding cuts on medical studies?
Halted research due to funding cuts can significantly disrupt ongoing medical studies, jeopardizing participant safety, delaying results, and eroding public trust in the research community. IRBs are unable to approve new sites or studies, limiting the progress and collaborative efforts necessary for advancing medical research.
Why is patient safety a primary concern for IRBs in research oversight?
Patient safety is a primary concern for IRBs because they are responsible for ensuring that all research participants are adequately informed about risks and benefits, and that their rights are protected. The role of IRBs is to prevent any potential harm during studies, which is crucial for maintaining ethical standards in medical research.
What collaboration exists between IRBs and federal agencies in research oversight?
IRBs work closely with federal agencies like the NIH to ensure compliance with federal regulations governing medical research. This collaboration includes the adoption of policies that require single IRB reviews for multi-site studies, which enhances the efficiency and ethical oversight of research involving human participants.
| Key Points | Details |
|---|---|
| Disruption of Oversight | The funding freeze disrupts the SMART IRB, impacting oversight of multi-site medical research. |
| Impact on Patient Safety | Cuts jeopardize the rights and safety of patients involved in medical studies, emphasizing the crucial role of IRBs. |
| Critical Role of IRBs | IRBs must review and oversee research proposals to protect participants, ensuring compliance with regulations. |
| Historical Context | IRBs arose from past ethical violations to maintain participant safety and trust, against backdrop of historical abuses. |
| Consequences of Funding Cuts | Ongoing research cannot expand, delaying studies, damaging trust, and risking participant health and safety. |
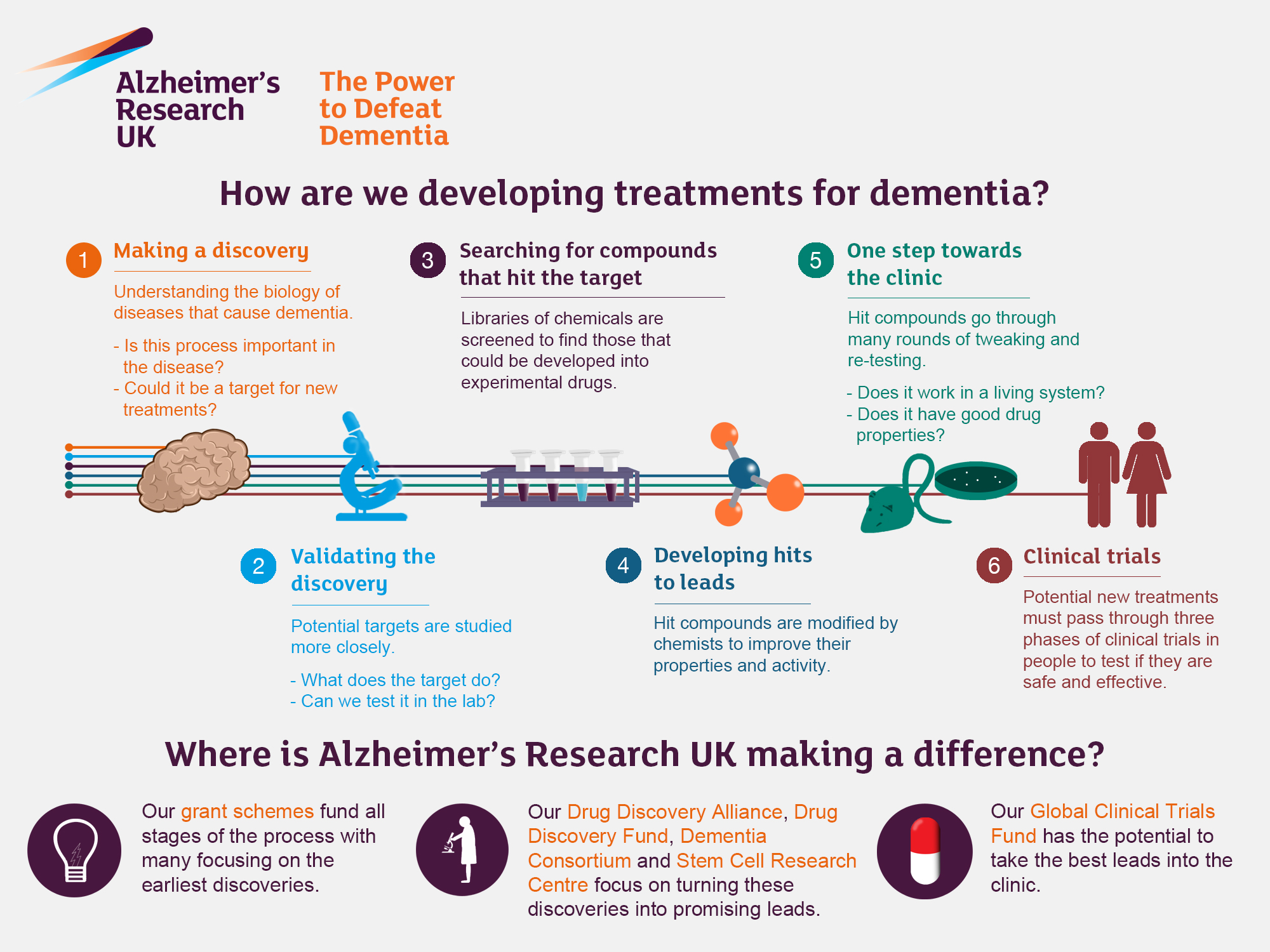
| Future of Research Collaboration | Without funding, collaborative research needed to innovate therapies, such as for Alzheimer’s, is severely hampered. |
Summary
Medical research oversight is crucial for ensuring the safety and rights of patients participating in clinical studies. The recent cuts to federal research funding underscore the importance of maintaining robust oversight systems like IRBs, which protect participants and uphold ethical standards in research. By facing obstacles in funding, research institutions risk compromising patient safety and trust, highlighting the need for continued investment and support in medical research oversight.