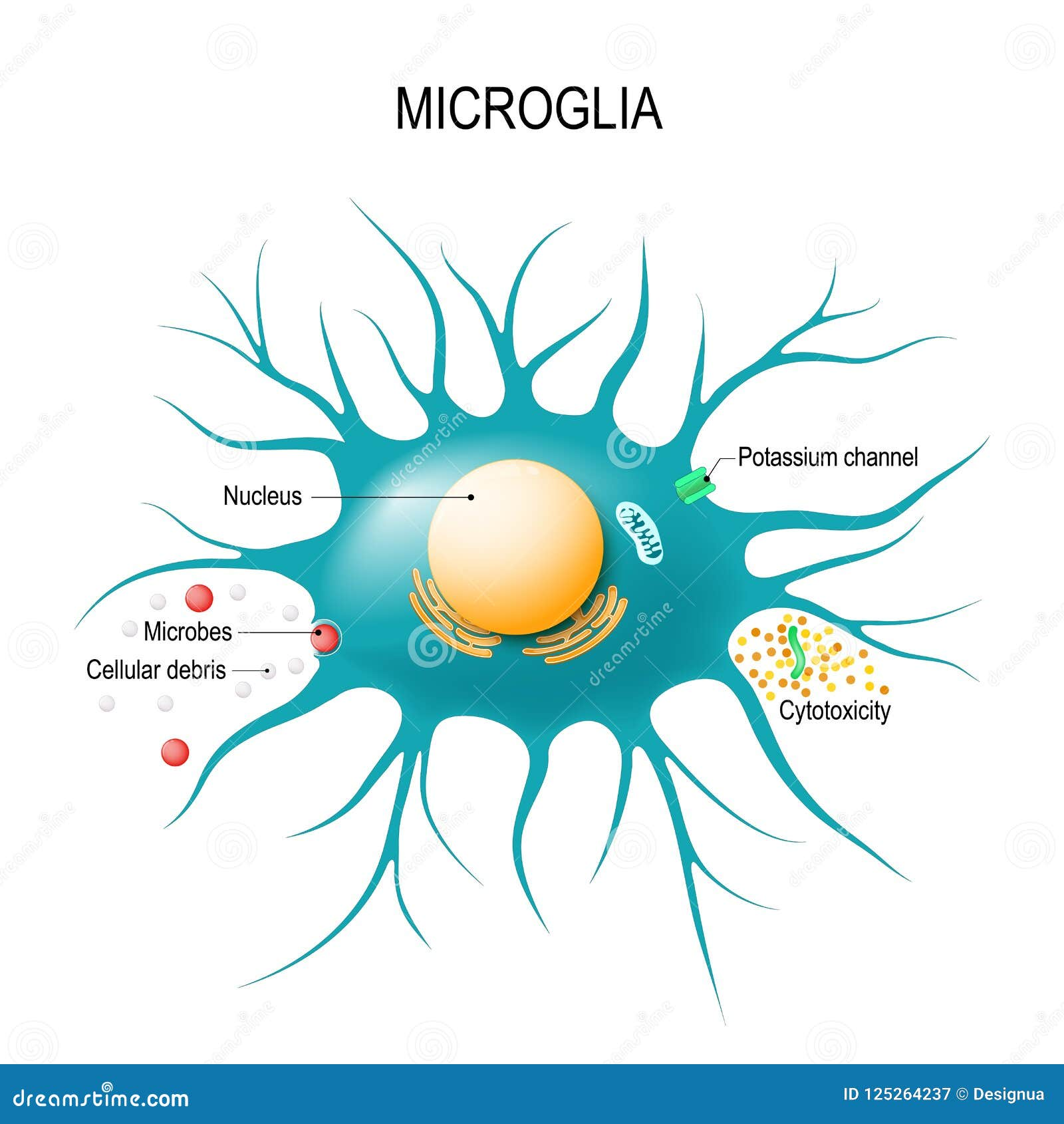
Microglial cells, the brain’s resident immune warriors, play a pivotal role in maintaining neurological health, especially in the context of Alzheimer’s disease and other neurodegenerative diseases. These specialized cells are responsible for patrolling the brain and engaging in crucial tasks like synaptic pruning, which is essential for the development and refinement of neural connections. However, as highlighted by Beth Stevens’s groundbreaking research, microglial dysfunction can lead to harmful outcomes such as excessive synaptic elimination, contributing to the pathology of Alzheimer’s and similar disorders. By understanding the complex mechanisms underlying microglial activity, scientists hope to pave the way for innovative treatments that could alleviate the burdens faced by millions afflicted with these conditions. Stevens’s work underscores the importance of continued research into our brain’s immune system, revealing how microglial cells may hold the key to unlocking future therapeutic strategies.
The brain’s cellular protectors, known as microglia, serve a critical function as the immune system within our central nervous system. These cells are essential for monitoring brain health, clearing out damaged cells, and pruning synapses, processes that are vital for proper cognitive function and neural communication. In her influential studies, Beth Stevens has shed light on the paradox of how these immune cells can contribute to neurodegenerative diseases like Alzheimer’s disease when their functioning goes awry. Her research emphasizes the significance of understanding how microglial cells interact with neurons, as these insights could lead to new methods for diagnosing and treating various brain disorders. By diving deeper into the world of microglia, researchers are uncovering potential pathways for therapeutic advancements in the realm of neurological health.
Understanding Microglial Cells and Their Role in Alzheimer’s Disease
Microglial cells, often referred to as the brain’s immune system, are pivotal in maintaining neurological health. These cells are responsible for monitoring the brain’s environment, removing damaged cells, and performing a critical function known as synaptic pruning. When operating optimally, microglia ensure that synapses — the connections between neurons — are efficiently maintained, thus facilitating proper communication across the brain. However, in conditions like Alzheimer’s disease, this process can become dysregulated. Aberrant synaptic pruning, as demonstrated in Beth Stevens’ research at Boston Children’s Hospital, exacerbates neurodegenerative diseases, leading to further cognitive decline among patients.
Beth Stevens’ transformative work underscores the dual role that microglial cells play in both protecting and potentially damaging brain health. While they are essential for cleaning up neuronal debris and supporting synaptic efficiency, their malfunction can contribute to the development of neurodegenerative diseases such as Alzheimer’s and Huntington’s disease. The insights gained from understanding these immune responses in the brain not only enhance our knowledge of disease processes but also pave the way for developing innovative therapies aimed at modulating microglial actions, thereby offering hope for millions affected by such debilitating conditions.
The Significance of Synaptic Pruning in Neurodegenerative Diseases
Synaptic pruning is a crucial developmental process where unnecessary or weak synaptic connections are eliminated, allowing for the refinement and optimization of neuronal circuits. This process is vital for cognitive development, yet its dysregulation is associated with neurodegenerative diseases, particularly Alzheimer’s disease. Research led by Beth Stevens has revealed that improper pruning mechanisms can significantly impact synaptic integrity and neuronal function, leading to the progressive loss of cognitive abilities characteristic of Alzheimer’s. Understanding these pathways is essential for identifying potential therapeutic targets that could mitigate synaptic loss.
The role of synaptic pruning extends beyond mere removal of excess synapses; it also involves the fostering of synaptic strength that is critical for learning and memory. In the context of neurodegenerative diseases, scientific inquiries into how microglial cells engage in synaptic pruning unlock possibilities for early intervention strategies. Innovations in targeting the signaling pathways of microglia and understanding their alterations in disease states could lead to new biomarkers for early detection and pave the way for therapeutics aimed at restoring synaptic health and ensuring sustained cognitive function in aging populations.
The Link Between Microglial Dysregulation and Neurodegeneration
Neurodegenerative diseases are often marked by a complex interplay of neuroinflammation and impaired synaptic function, with microglial cells at the center of this process. Research from investigators like Beth Stevens highlights how aberrant microglial activity contributes to the progression of Alzheimer’s disease by promoting neuroinflammation and facilitating synaptic loss. These insights stress the importance of understanding microglial biology as a way to better comprehend the pathological mechanisms underlying neurodegenerative conditions. Addressing microglial dysfunction may unveil new avenues for neuroprotective strategies.
The findings from the Stevens Lab suggest that interventions targeting microglial activity could potentially halt or even reverse the progress of neurodegenerative diseases. By stimulating proper microglial function and promoting healthy synaptic pruning, researchers aim to develop therapies that not only address existing symptoms of Alzheimer’s but also modify disease trajectory. With growing awareness of the brain’s immune landscape, therapeutic strategies may soon involve modulating microglial responses, assisting in the maintenance of cognitive function in those affected by neurodegenerative disorders.
Beth Stevens’ Groundbreaking Research on Neuroinflammation
Beth Stevens’ contributions to understanding neuroinflammation have significantly advanced the field of neurology, particularly in the context of Alzheimer’s and related disorders. Her research highlights how microglial cells, as part of the brain’s immune system, engage in both protective and harmful roles. By following pathophysiological mechanisms of disease progression closely, Stevens’ work has established critical links between neuroinflammation and cognitive decline, emphasizing the necessity of targeting inflammation in therapeutic strategies.
As Stevens’ studies show, the role of neuroinflammation in Alzheimer’s disease is complex and multifaceted. While inflammation can serve as a protective response to injury, chronic activation of microglia leads to detrimental effects, perpetuating neurodegeneration. Innovations stemming from Stevens’ research advocate for the development of anti-inflammatory therapies that can intervene at various points in this pathological cascade, holding promise not only for Alzheimer’s but for a broader spectrum of neurodegenerative diseases.
From Curiosity-Driven Research to Clinical Implications
The journey of scientific discovery often begins with curiosity-driven research, a notion that Beth Stevens embodies in her work. Early explorations into the role of microglial cells revealed critical insights into how these cells contribute to synaptic health and immunity in the brain. As state-of-the-art techniques have emerged, Stevens has leveraged these advancements to translate findings from basic science into applicable clinical implications, bridging the gap between laboratory research and patient care.
The nexus of foundational research and clinical application is vital for developing effective treatments for neurodegenerative diseases. Stevens’ emphasis on the role of microglia and their interactions in disease offers a template for future research endeavors. By fostering an environment where scientists can pursue ambitious inquiries without immediate clinical goals, breakthroughs like those in the understanding of microglial functions become possible, yielding the potential to change lives through novel therapeutic options.
Innovative Biomarkers in Alzheimer’s Disease Research
A pivotal challenge in Alzheimer’s disease is the development of reliable biomarkers that can indicate disease pathology and progression. Research from Stevens’ lab has shown that understanding microglial behavior can uncover potential biomarkers that reflect inflammation and synaptic alterations in the brain. These biomarkers hold the promise of enabling earlier diagnosis and more tailored treatment strategies, significantly improving patient outcomes.
As scientists continue to delve into the mechanisms of microglial activity, there is an anticipation that novel biomarkers will not only facilitate diagnostics but also aid in monitoring treatment efficacy in clinical settings. Initiatives that explore the relationship between microglial dysfunction and specific biomarkers are crucial in advancing our comprehension of Alzheimer’s pathology. Such innovations could revolutionize approaches to management, enabling proactive interventions that can slow or prevent cognitive decline.
The Future of Neurodegenerative Disease Therapies
Looking ahead, the future of therapies for neurodegenerative diseases, particularly Alzheimer’s, hinges on a deeper understanding of the brain’s immune responses and functions of microglial cells. The research spearheaded by Beth Stevens offers a hopeful glimpse into what may be possible through the targeted modulation of microglial activity and enhanced synaptic resilience. The development of pharmacological agents that can fine-tune microglial actions could usher in a new era of treatment for Alzheimer’s and related conditions.
The promise of innovative therapies rests on the foundation laid by exploration into the groundwork of neurobiology. As the scientific community continually refines our understanding of microglial roles and mechanisms in neurodegeneration, we may witness breakthroughs that not only enhance the quality of life for Alzheimer’s patients but potentially delay the onset of cognitive decline. The collaborative efforts between researchers, clinicians, and funding bodies are crucial to driving this essential research forward.
Impact of Federal Funding on Neuroscience Research
Federal funding has been a cornerstone for transformative research in various fields, especially in neuroscience. As highlighted by Beth Stevens, NIH support has been instrumental in not only advancing studies on neurodegenerative diseases but also in laying the groundwork for understanding the fundamental principles of brain immunity and microglial function. The sustained investment in such research is essential for fostering breakthroughs that can ultimately lead to effective therapeutic interventions.
The role of federal grants cannot be overstated; they provide researchers the resources necessary to pursue ambitious projects that might otherwise be deemed too risky or unfocussed. By facilitating access to cutting-edge technology and collaborative networks, these funds catalyze significant discoveries in neurobiology, enhancing our capacity to combat Alzheimer’s disease and improve outcomes for those affected by neurodegeneration. Continued advocacy for and emphasis on research funding is critical to motivating future generations of scientists to confront the challenges posed by these debilitating illnesses.
Ethical Considerations in Neurodegenerative Disease Research
As the field of neuroscience advances, ethical considerations must be at the forefront of research, particularly in studies involving vulnerable populations affected by Alzheimer’s disease. Ethical guidelines help ensure that research practices in neurodegenerative disease are conducted with integrity, prioritizing the dignity and rights of participants. Beth Stevens’ emphasis on transparency and responsible conduct sets a precedent for maintaining ethical standards in the pursuit of scientific knowledge.
Additionally, the implications of research outcomes extend beyond the laboratory, urging scientists to consider how findings will influence patient care and public health policy. As new biomarkers and therapeutic strategies are developed, engaging with ethical frameworks ensures that advancements in neuroscience translate into equitable and accessible treatments for all individuals facing the challenges of Alzheimer’s and neurodegenerative diseases. The interplay between research and ethics is indispensable for fostering trust and collaboration between scientists, patients, and medical professionals.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease?
Microglial cells are the brain’s immune system and play a crucial role in Alzheimer’s disease by monitoring for illness and removing damaged cells. However, in Alzheimer’s, their function can become abnormal, leading to excessive synaptic pruning, which may contribute to neurodegeneration.
How do microglial cells contribute to synaptic pruning in the brain?
Microglial cells contribute to synaptic pruning by selectively eliminating weak or unused synapses during brain development and in disease states. This process is essential for normal brain function but can be disrupted in neurodegenerative diseases like Alzheimer’s, resulting in cognitive decline.
How does Beth Stevens’ research advance our understanding of microglial cells in neurodegenerative diseases?
Beth Stevens’ research has transformed our understanding of microglial cells by elucidating their role in synaptic pruning and their involvement in neurodegenerative diseases such as Alzheimer’s. Her findings may lead to the development of new biomarkers and therapies targeting microglial dysfunction.
What is the significance of microglial activation in neurodegenerative diseases?
Microglial activation is significant in neurodegenerative diseases because it indicates an immune response to injury or disease within the brain. In conditions like Alzheimer’s, chronic activation can exacerbate neurodegeneration through excessive synaptic pruning and inflammation.
Can targeting microglial cells lead to new treatments for Alzheimer’s disease?
Yes, targeting microglial cells offers a promising avenue for new treatments for Alzheimer’s disease. By modulating their activity, researchers aim to correct dysfunctional pruning and control inflammation, which could slow down the progression of neurodegenerative disorders.
What funding has supported research on microglial cells and Alzheimer’s disease?
Research on microglial cells and Alzheimer’s disease has been significantly supported by federal funding agencies like the National Institutes of Health (NIH), which provide essential resources for advancing our understanding of brain immune mechanisms and developing therapeutic strategies.
What are some potential biomarkers related to microglial cells in Alzheimer’s disease?
Potential biomarkers related to microglial cells in Alzheimer’s disease include specific inflammatory markers and molecules associated with synaptic pruning, which can help in early diagnosis and in monitoring disease progression or treatment efficacy.
What findings have emerged from Beth Stevens’ lab regarding microglial dysfunction in brain health?
Findings from Beth Stevens’ lab indicate that microglial dysfunction, characterized by abnormal synaptic pruning, contributes to various neurodegenerative diseases, including Alzheimer’s. This research highlights the importance of microglia in maintaining brain health and its potential as a therapeutic target.
How does microglial dysfunction relate to neurodegenerative conditions beyond Alzheimer’s?
Microglial dysfunction is implicated in several neurodegenerative conditions, beyond Alzheimer’s, such as Huntington’s disease. Poor regulation of microglial activity can lead to inappropriate synaptic pruning and neuroinflammation, exacerbating neuronal damage and cognitive decline.
What future research directions are there for understanding microglial cells in brain diseases?
Future research directions for understanding microglial cells include exploring their molecular signaling pathways, developing methods to modulate their function therapeutically, and identifying how their role varies across different neurodegenerative diseases, including Alzheimer’s.
| Key Points | Details |
|---|---|
| Microglial Cells’ Role | Act as the brain’s immune system, clearing damaged cells and pruning synapses. |
| Link to Alzheimer’s Disease | Aberrant pruning by microglia can contribute to Alzheimer’s and other neurodegenerative diseases. |
| Research Background | Beth Stevens’ curiosity-driven research at Boston Children’s Hospital led to new insights about microglial cells. |
| Importance of Funding | Federal funding has been crucial in advancing our understanding of microglial cells and their role in disease. |
| Potential for Treatments | Research on microglia may lead to new biomarkers and medications for treating Alzheimer’s disease. |
Summary
Microglial cells play a critical role in maintaining brain health and understanding neurodegenerative diseases like Alzheimer’s. Their function as the immune system of the brain includes monitoring for damage and clearing debris, which is essential for neurological health. Research led by Beth Stevens has illuminated how dysfunctional microglial activity can be linked to Alzheimer’s disease and similar disorders. Through curiosity-driven science supported by federal funding, the Stevens Lab has opened pathways for new biomarkers and therapies that could enhance care for those affected by these debilitating diseases.