Gene therapy for hemophilia represents a groundbreaking advancement in treating this challenging bleeding disorder, offering hope for those who have long faced the burden of constant worry and daily injections. Recently, the FDA’s approval of Hemgenix has spurred a new wave of optimism, particularly among patients and families seeking effective hemophilia B treatment. As we uncover gene therapy success stories, it’s clear that the benefits of gene therapy extend beyond mere survival; they offer the promise of a normal life without the specter of frequent bleeding episodes. For many living with hemophilia, this innovative approach symbolizes a potential cure, changing how they might navigate their condition. As researchers continue to explore the landscape of genetic medicine, the journey from managing symptoms to embracing a healthier, more liberated future is gaining unprecedented momentum.
Innovative treatments like gene therapy for hemophilia have transformed the landscape of medical care for individuals affected by bleeding disorders. Alternative treatments are now being explored that leverage advanced genetic technologies to correct the underlying mutations responsible for hemophilia B, making the concept of a cure more tangible than ever. The recent success of Hemgenix, with its promising results and benefits, shines a spotlight on the evolving possibilities within hemophilia care. Patients no longer have to be defined solely by their condition; they can envision a life free from the constraints of traditional therapy. As stories of triumph emerge, the horizon appears bright for those facing the challenges of this condition.
Understanding Hemophilia and Its Challenges
Hemophilia is a genetic disorder that impairs the blood’s ability to clot, leading to excessive bleeding from even minor injuries. The condition predominately affects males due to its inheritance pattern linked to the X chromosome. Patients with hemophilia experience spontaneous bleeding episodes, which can occur internally or externally, leading to severe consequences if not managed properly. Throughout history, individuals like Terence Blue have faced numerous challenges in living with hemophilia. The fear of bleeding and the need for regular infusions of clotting factors can result in substantial limitations on physical activities and social interactions.
Innovations in hemophilia treatments have significantly improved patients’ quality of life. Prophylactic therapies, introduced in the past few decades, have led to better management of the condition. Yet, many patients still deal with the psychological and physical impacts of hemophilia. The ongoing developments in treatment options, such as synthetic factors and gene therapies, show hope for more reliable management of the disease, ultimately leading to a future where living with hemophilia involves fewer restrictions.
Gene Therapy for Hemophilia: A Revolution in Treatment
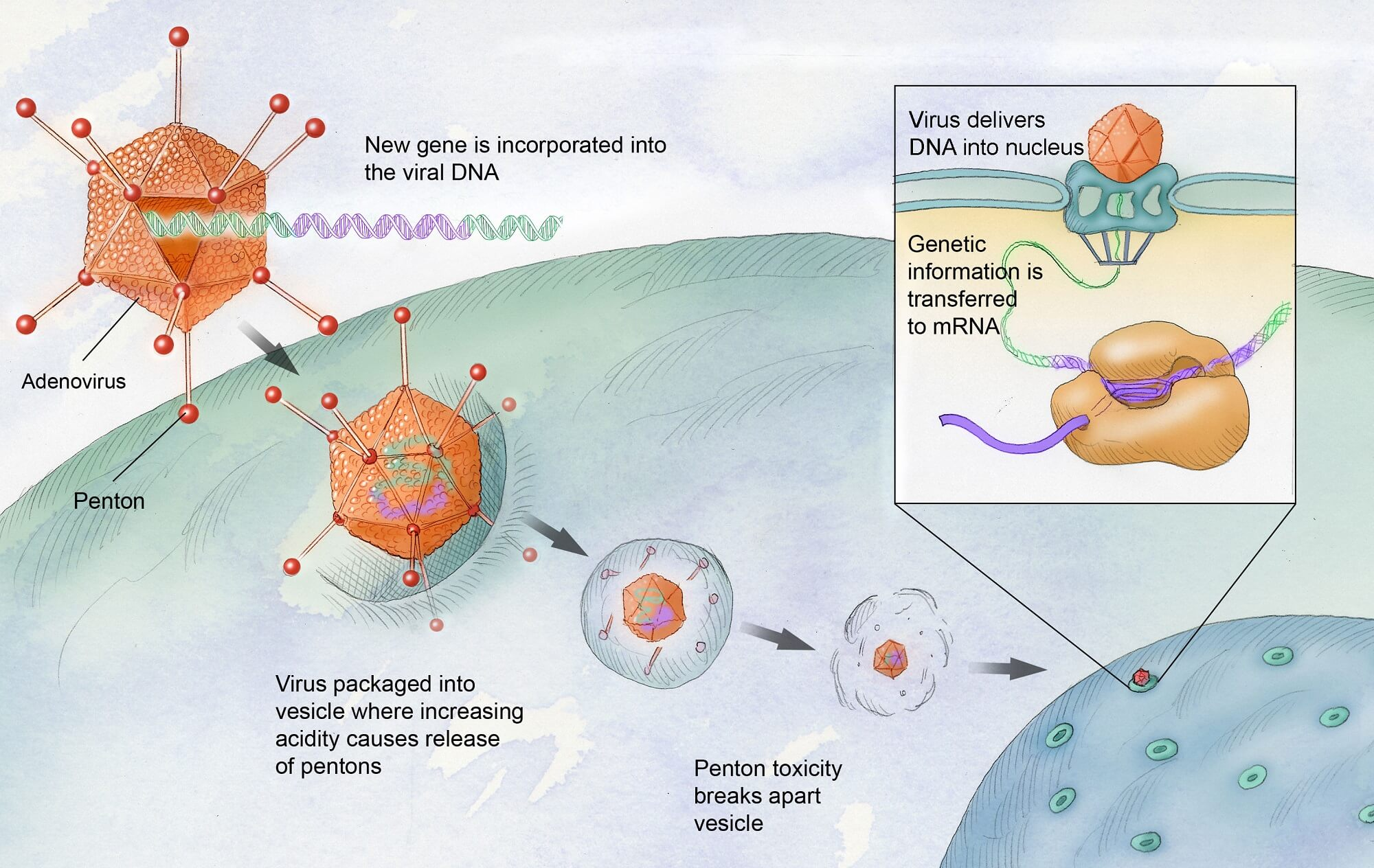
Gene therapy for hemophilia represents a groundbreaking approach that has the potential to transform patients’ lives fundamentally. A pioneering treatment called Hemgenix, which was recently approved by the FDA, exemplifies this radical shift. By introducing corrective genes into a patient’s liver cells, Hemgenix aims to restore the production of clotting factor IX, the protein lacking in hemophilia B patients. Early results from clinical trials have been promising, with many patients not requiring regular infusions of clotting factors for prolonged periods after treatment.
The benefits of gene therapy are profound, offering not just a reduction in bleeding episodes but also improving overall quality of life. Patients like Terence Blue, who recently received the gene therapy, are experiencing significant changes. Rather than relying on frequent injections, they may enjoy more freedom and peace of mind, engaging in activities without the looming fear of bleeding. As gene therapy continues to advance, it raises the prospect of long-lasting, even possibly curative solutions for hemophilia patients.
The Impact of FDA Approval on Gene Therapies
The FDA approval of gene therapies signifies a monumental step forward in the treatment of hemophilia. With the endorsement of Hemgenix, patients now have access to a one-time treatment option instead of ongoing, traditional therapies. This new approach has gained considerable attention not only for its innovative mechanism of delivering healthy genes but also for its implications regarding patient adherence to treatment regimens. The approval not only validates years of research but also empowers patients with more effective treatment choices, enhancing their experience and outlook toward living with hemophilia.
However, the success of gene therapy hinges on more than just regulatory approval. Market acceptance and healthcare infrastructure play critical roles in determining accessibility. With treatments like Hemgenix coming with a hefty price tag, $3.5 million per treatment, discussions surrounding insurance coverage and patient access have become essential. For hemophilia patients, the promise of cutting-edge therapy is accompanied by concern over financial barriers that may impede the wide acceptance of life-altering treatments.
The Success Stories of Gene Therapy in Hemophilia
As gene therapies like Hemgenix enter the clinical landscape, numerous success stories are beginning to emerge, showcasing the potential of this innovative treatment approach. Patients previously burdened by the daily rituals of injections and constant monitoring of their condition are finding newfound excitement in their health outcomes. The transformative effects of gene therapy are not just limited to medical improvement; they provide newfound freedom to pursue desires and passions that were once hindered by their hemophilia.
These success stories foster a growing optimism among patients and families affected by hemophilia. For many individuals, the reality of having a single treatment that could reduce or eliminate the need for regular clotting factor infusions is both a dream come true and a significant advancement in hemophilia B treatment options. As more patients share their experiences, they help to inspire a broader acceptance of gene therapy as a viable and beneficial treatment modality in dia_today’s healthcare ecosystem.
Living with Hemophilia: The Emotional Toll
Living with hemophilia comes with profound emotional and psychological challenges. The constant anxiety surrounding the potential for bleeding episodes can affect mental health and lead to feelings of isolation. Patients often find themselves needing to explain their condition to friends and family, which can create barriers in social relationships. Over years, hemophilia can significantly alter the way individuals engage with the world around them, leading to a desire for social acceptance and understanding.
As new treatments, like gene therapy, emerge, there is hope for a future where individuals with hemophilia can live with less fear and more confidence. Stories shared by patients, including the progress made from innovations in treatments, demonstrate that the battle with hemophilia is not solely a physical one. The emotional aspects are paramount, highlighting a need for emotional support and resources for patients and their families. Addressing both physical and emotional challenges remains important for improving the overall quality of life for those living with hemophilia.
Market Challenges for Gene Therapies
Despite the groundbreaking advancements represented by gene therapies for hemophilia, these treatments face significant market challenges that may impact their accessibility and success. Pricing models for gene therapies, like Hemgenix, have sparked considerable debate within the healthcare community. With treatments aspiring to correct genetic aberrations at a high cost, the burden falls on healthcare systems to negotiate pricing with pharmaceutical companies while ensuring that patients receive the best possible care.
Additionally, acceptance and awareness among patients are crucial for the widespread adoption of these therapies. The experience of previous gene therapies, such as Pfizer’s Beqvez, illustrates that, despite FDA approval, market realities can complicate the dynamics between patients, providers, and payers. The healthcare community must collectively address these challenges to facilitate patient access and ensure that advancements in gene therapy translate into positive outcomes for those affected by hemophilia.
The Future of Hemophilia Treatments
Looking to the future, the landscape of hemophilia treatments is evolving rapidly, with numerous gene and cell therapies in development. Research is progressing towards more targeted approaches, aiming to enhance the efficacy and safety profiles of treatments aimed at correcting the genetic mutations associated with hemophilia. With an overarching focus on improving patient experiences and long-term health outcomes, the future holds promise for advancements that may render hemophilia a manageable condition rather than a lifelong burden.
Moreover, the dialogue surrounding gene therapy continues to expand, encouraging collaboration within the research community, healthcare providers, and patient advocates. These collaborations aim to overcome barriers to access, increase awareness among patients, and reinforce the support systems critical for those living with hemophilia. Ensuring that innovations reach those in need while addressing market and pricing challenges will be essential for transforming hemophilia care and improving the lives of patients significantly.
Navigating Life Post-Gene Therapy
For patients who have undergone gene therapy, such as Terence Blue, the transition to life after treatment involves a journey of adjustment and anticipation. The reduction of traditionally required clotting factor injections can present a unique challenge in adapting to this new reality. As these patients navigate their post-treatment lives, they may find themselves reevaluating their daily routines and the risks that once dominated their lives due to hemophilia.
Ultimately, the process of acclimatization to life post-gene therapy signifies more than just an improvement in physical health; it embodies a new outlook and set of opportunities. Adjusting to a reduced reliance on traditional treatments allows patients to engage more fully with their lives and pursue interests previously limited by their condition. This emotional and psychological transition is just as vital as the physical healing they experience, with a potential long-lasting impact on their quality of life.
Community Support and Awareness for Hemophilia
Community support plays a crucial role in improving the lives of those living with hemophilia. As increased awareness of gene therapies and their potential benefits spreads, the conversation around hemophilia is evolving, encouraging more comprehensive support for patients and their families. Local and national organizations are focusing on outreach and education, ensuring that individuals understand their treatment options and the support services available to them.
Additionally, empowering families and communities to facilitate discussions about hemophilia promotes a greater understanding of the condition as a whole. Shared experiences, like those of patients who have undergone gene therapy, can help demystify the treatment and encourage others to explore these options. By fostering openness and connectivity, the hemophilia community can provide the emotional support needed to navigate the complexities of treatment and living with this condition.
Frequently Asked Questions
What is gene therapy for hemophilia B, and how does Hemgenix work?
Gene therapy for hemophilia B, such as Hemgenix, involves delivering a corrected copy of the gene responsible for producing clotting factor IX into the patient’s liver. This is achieved using a modified virus that acts as a vector to insert the gene, facilitating the body’s ability to produce the missing clotting factor. Hemgenix, which received FDA approval, has demonstrated promising results in clinical trials, showing that many patients do not require traditional prophylactic treatments post-therapy.
What are the benefits of gene therapy for hemophilia compared to traditional treatments?
The benefits of gene therapy for hemophilia include potentially long-lasting effects that may reduce or eliminate the need for regular clotting factor infusions. This approach offers a significant lifestyle improvement by reducing the anxiety and burden associated with frequent treatments. For example, Hemgenix has shown effectiveness in raising factor IX levels in patients, resulting in fewer bleeding episodes and improved quality of life.
Are there any success stories related to gene therapy for hemophilia B?
Yes, there are notable success stories from patients who have undergone gene therapy for hemophilia B, particularly with Hemgenix. Terence Blue, the first patient in New England to receive this therapy, reported a remarkable improvement in his healing and overall health, with significant increases in his factor IX levels following the treatment. Clinical results show that a majority of participants in trials have not needed additional factor IX infusions for years after receiving Hemgenix.
What is the FDA approval status of Hemgenix for hemophilia treatment?
Hemgenix received FDA approval in November 2022, marking a significant advancement in gene therapy for hemophilia B. This approval underscores the therapy’s potential to transform the management of this condition, moving from traditional reliance on clotting factor infusions to a more permanent solution.
How does living with hemophilia change with the advent of gene therapy?
Living with hemophilia may shift dramatically with gene therapy advancements like Hemgenix. Patients may experience fewer spontaneous bleeding episodes and reduced treatment frequency, allowing for a more normal lifestyle. Patients report less anxiety related to their condition, enhanced healing times, and greater overall health post-treatment.
What challenges do gene therapy treatments like Hemgenix face in the market?
While gene therapies like Hemgenix are promising, they face challenges such as high treatment costs, reaching effective pricing levels for widespread adoption, and ensuring sufficient patient and physician acceptance. The experience of other therapies being withdrawn from the market highlights the need for a sustainable demand and support system in healthcare to fully realize the benefits of these innovative treatments.
What should patients expect when considering gene therapy for hemophilia?
Patients considering gene therapy for hemophilia should expect a thorough evaluation process, discussions about potential risks and benefits, and preparation for a one-time treatment. They can look forward to a significant change in their hemophilia management, as many patients report substantial and sustained increases in clotting factor levels post-therapy, as evidenced by clinical trials.
| Key Point | Description |
|---|---|
| Terence Blue’s Story | Terence Blue received a new gene therapy for hemophilia B, marking a significant change in his life after managing the condition for 27 years. |
| What is Gene Therapy? | Gene therapy, such as Hemgenix, aims to correct disease-causing mutations by introducing a corrected gene into the patient’s cells, offering long-term benefits with a single treatment. |
| FDA Approval | Hemgenix was approved by the FDA in November 2022, making it the first therapy of its kind for hemophilia B. |
| Market Challenges | Despite potential benefits, gene therapies face market pressures that can affect their availability and affordability. |
| Cost of Treatment | The cost for Blue’s treatment is approximately $3.5 million, highlighting the financial hurdle of gene therapies. |
| Long-term Effects | Patients receiving gene therapies like Hemgenix may experience long-lasting benefits, with some not needing daily treatments after administration. |
| President’s Optimism | Expert opinions reflect optimism that gene therapies will continue to evolve and effectively treat various conditions including hemophilia. |
Summary
Gene therapy for hemophilia represents a transformative step in treating this condition. By utilizing advanced technology to correct the underlying genetic causes of hemophilia B, therapies like Hemgenix provide patients hope for a life free from the burdens of daily treatment and the constant risk of bleeding. The journey of patients like Terence Blue illustrates not only the advancements in medical science but also the potential for a brighter future for those affected by hemophilia.